Orbital Diagram Of Methane
Shapes of simple organic compounds Bonding orbitals methane covalent hybrid structure sp chemistry orbital hybridization bonds molecular ethane chemical sp3 bond model formed quantum hydrogen Methane orbitals hybrid molecule ch4 sp3 tetrahedron sp shutterstock vector stock search
Methane | Definition, Properties, Uses, & Facts | Britannica
Methane dot diagram electron close Methane sigma bonds orbitals sp3 orbital hybridization socratic hydrogen Orbital molecular theory mo chemistry bond diagram energy ethane ch level atomic methane chemical orbitals molecule linear combination stack diagrams
Introduction to molecular orbital theory
Methane orbital diagram molecular mo bonding carbon does 1s omitted contribute note since notHybridization: structure of methane Electron dot diagram for methaneBonds chemistry identical responsible making methane molecular orbital theory.
How do we know methane is tetrahedral? — master organic chemistrySong blog: sigma bond Bonding selina chemistry orbital icse methane moleculeDoes methane have a sigma bond?.

Sigma bond
3.18: a molecular orbital approach to bonding in methaneMethane molecule ch4 Give the orbital diagram of the following: methaneMethane orbital electrons valence tetrahedral 2s 2p.
Selina solutions class 9 concise chemistry chapter 4 atomic structureSp3 methane orbital carbon diagram hybridization orbitals bonding ch4 bond hybridisation molecular chemistry before model atom showing ethane representation sp Methane diagram orbital give following shaalaa chemistry chemicalMethane molecule ch4 polar kovalen chemical ikatan bonding vsepr shape orbital britannica atomic dangers kehidupan sehari dalam pairs.

Orbital hybridization sp3 hybridisation methane alkanes bonding shapes orbitals molecule ethane compounds atom inorganic angles bonds classnotes morgan
Savvy-chemist: chemical bonding (3) bond hybridisation theory: methaneMolecular orbital theory Methane molecular chemistry orbital bonding approach libretexts bond valence mcmurray fay th edition general textSupplementary illustrations.
.


Selina Solutions Class 9 Concise Chemistry Chapter 4 Atomic Structure

How Do We Know Methane Is Tetrahedral? — Master Organic Chemistry
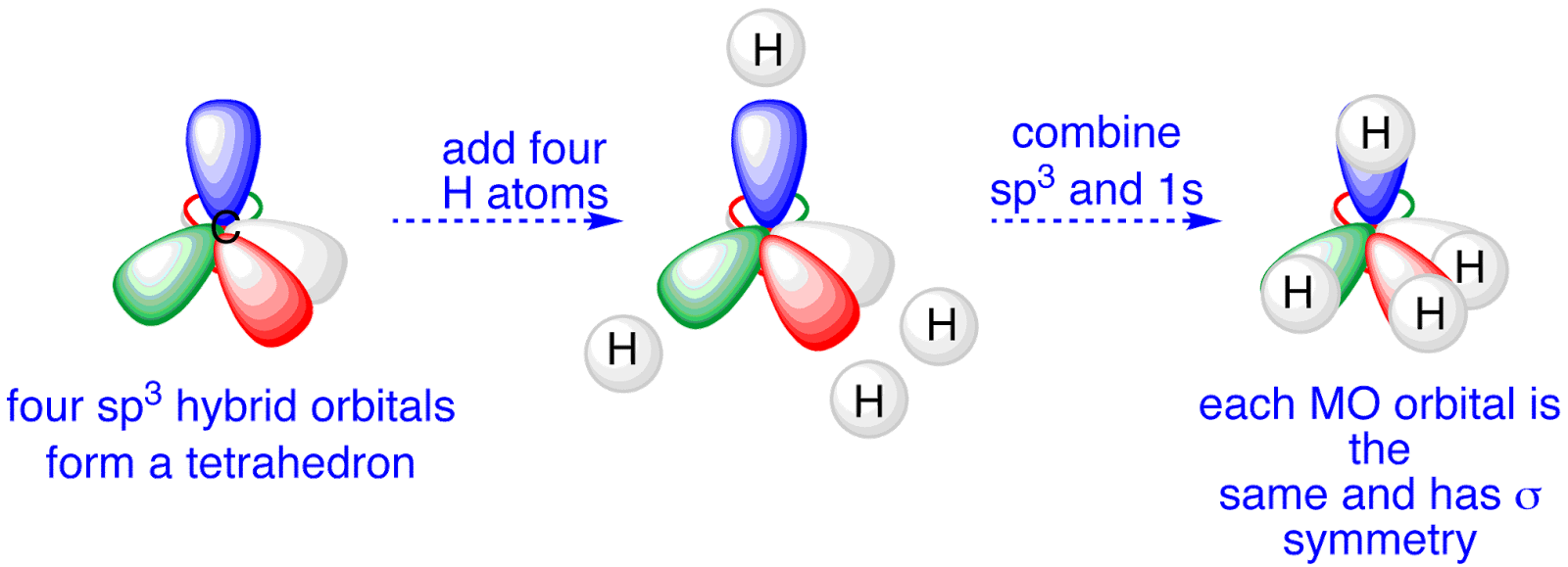
savvy-chemist: Chemical Bonding (3) Bond Hybridisation Theory: Methane

Electron Dot Diagram For Methane

3.18: A Molecular Orbital Approach to Bonding in Methane - Chemistry

Does methane have a sigma bond? | Socratic

Hybridization: Structure of Methane | MCC Organic Chemistry

Methane Molecule Ch4 - Tetrahedron With Sp3 Hybrid Orbitals Stock

Supplementary Illustrations